Embark on an enthralling journey into the realm of organic chemistry with our captivating Class of Organic Compounds Crossword. Dive into the diverse world of these fundamental building blocks of life, unraveling their intricate structures, properties, and myriad applications.
From the simplest alkanes to the complex biomolecules that govern our very existence, organic compounds orchestrate a symphony of chemical reactions that shape our world. Prepare to be captivated as we delve into their classification, identification, reactivity, and the profound impact they have on our daily lives.
Organic Compound Classification
Organic compounds are an extensive group of chemical substances that contain carbon atoms. They form the basis of all living organisms and play a crucial role in various biological processes. Organic compounds are classified into different classes based on their functional groups, which are specific arrangements of atoms within the molecule that determine their chemical properties and reactivity.
The International Union of Pure and Applied Chemistry (IUPAC) has established a systematic nomenclature system for naming organic compounds. This system assigns unique names to each compound based on its structure and functional groups. The IUPAC nomenclature rules provide a standardized way of identifying and communicating about organic compounds, facilitating clear and unambiguous scientific communication.
Functional Groups
Functional groups are the key structural features that distinguish different classes of organic compounds. Each functional group consists of a specific arrangement of atoms, such as carbon, hydrogen, oxygen, nitrogen, or halogens, that imparts characteristic chemical properties to the molecule.
- Alkanes: Contain only carbon and hydrogen atoms, with single bonds between the carbon atoms.
- Alkenes: Contain at least one carbon-carbon double bond.
- Alkynes: Contain at least one carbon-carbon triple bond.
- Alcohols: Contain a hydroxyl group (-OH) attached to a carbon atom.
- Ethers: Contain an oxygen atom bonded to two carbon atoms.
- Aldehydes: Contain a carbonyl group (C=O) bonded to at least one hydrogen atom.
- Ketones: Contain a carbonyl group (C=O) bonded to two carbon atoms.
- Carboxylic acids: Contain a carboxyl group (-COOH), consisting of a carbonyl group bonded to a hydroxyl group.
- Esters: Contain an ester group (-COOR), consisting of a carbonyl group bonded to an oxygen atom, which is in turn bonded to an alkyl or aryl group.
- Amides: Contain an amide group (-CONH 2), consisting of a carbonyl group bonded to a nitrogen atom, which is in turn bonded to two hydrogen atoms.
IUPAC Nomenclature
The IUPAC nomenclature system for naming organic compounds follows a set of rules and guidelines. These rules consider the structure, functional groups, and substituents of the molecule to assign a unique and systematic name.
The IUPAC nomenclature system provides several advantages. It allows chemists to:
- Identify and describe organic compounds clearly and concisely.
- Predict the properties and reactivity of organic compounds based on their functional groups.
- Communicate about organic compounds in a standardized and unambiguous manner.
Common Classes of Organic Compounds
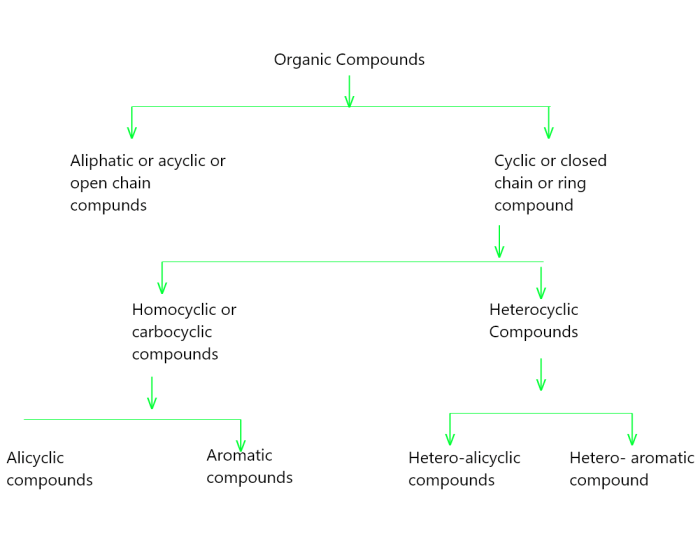
Organic compounds are a diverse group of chemical substances that contain carbon atoms. They are found in all living things and are the basis of many of the materials we use in everyday life, such as plastics, fuels, and pharmaceuticals.
Organic compounds can be classified into several different classes, based on their functional groups. Functional groups are atoms or groups of atoms that are attached to the carbon atoms in an organic molecule and determine its chemical properties.
Alkanes
Alkanes are a class of organic compounds that contain only carbon and hydrogen atoms. They are saturated hydrocarbons, meaning that all of the carbon atoms are bonded to four other atoms. Alkanes are nonpolar and have low boiling points.
Examples of alkanes include:
- Methane (CH 4)
- Ethane (C 2H 6)
- Propane (C 3H 8)
- Butane (C 4H 10)
- Pentane (C 5H 12)
Alkenes
Alkenes are a class of organic compounds that contain at least one carbon-carbon double bond. They are unsaturated hydrocarbons, meaning that not all of the carbon atoms are bonded to four other atoms.
Examples of alkenes include:
- Ethene (C 2H 4)
- Propene (C 3H 6)
- Butene (C 4H 8)
- Pentene (C 5H 10)
- Hexene (C 6H 12)
Alkynes
Alkynes are a class of organic compounds that contain at least one carbon-carbon triple bond. They are unsaturated hydrocarbons, meaning that not all of the carbon atoms are bonded to four other atoms.
Examples of alkynes include:
- Ethyne (C 2H 2)
- Propyne (C 3H 4)
- Butyne (C 4H 6)
- Pentyne (C 5H 8)
- Hexyne (C 6H 10)
Alcohols
Alcohols are a class of organic compounds that contain at least one hydroxyl group (-OH). They are polar and have relatively high boiling points.
Examples of alcohols include:
- Methanol (CH 3OH)
- Ethanol (C 2H 5OH)
- Propanol (C 3H 7OH)
- Butanol (C 4H 9OH)
- Pentanol (C 5H 11OH)
Aldehydes
Aldehydes are a class of organic compounds that contain a carbonyl group (-COH). They are polar and have relatively low boiling points.
Examples of aldehydes include:
- Formaldehyde (HCHO)
- Acetaldehyde (CH 3CHO)
- Propionaldehyde (C 2H 5CHO)
- Butyraldehyde (C 3H 7CHO)
- Valeraldehyde (C 4H 9CHO)
Ketones, Class of organic compounds crossword
Ketones are a class of organic compounds that contain a carbonyl group (-CO-) that is bonded to two carbon atoms. They are polar and have relatively low boiling points.
Examples of ketones include:
- Acetone (CH 3COCH 3)
- Methyl ethyl ketone (CH 3COC 2H 5)
- Diethyl ketone (C 2H 5COC 2H 5)
- Cyclohexanone (C 6H 10O)
- Camphor (C 10H 16O)
Carboxylic Acids
Carboxylic acids are a class of organic compounds that contain a carboxyl group (-COOH). They are polar and have relatively high boiling points.
Examples of carboxylic acids include:
- Formic acid (HCOOH)
- Acetic acid (CH 3COOH)
- Propionic acid (C 2H 5COOH)
- Butyric acid (C 3H 7COOH)
- Valeric acid (C 4H 9COOH)
Esters
Esters are a class of organic compounds that contain an ester group (-COOR). They are polar and have relatively low boiling points.
Examples of esters include:
- Methyl acetate (CH 3COOCH 3)
- Ethyl acetate (CH 3COOC 2H 5)
- Propyl acetate (CH 3COOC 3H 7)
- Butyl acetate (CH 3COOC 4H 9)
- Pentyl acetate (CH 3COOC 5H 11)
Organic Compound Identification
Organic compound identification is a crucial aspect of organic chemistry, enabling scientists to determine the structure and identity of unknown compounds. Various techniques are employed for this purpose, each with its unique principles and applications.
Spectroscopy
Spectroscopy involves the interaction of electromagnetic radiation with molecules, providing information about their molecular structure and composition.
- Infrared (IR) spectroscopy: IR radiation is absorbed by specific functional groups, providing insights into the presence of these groups within the molecule.
- Nuclear magnetic resonance (NMR) spectroscopy: NMR spectroscopy utilizes the magnetic properties of atomic nuclei, particularly 1H and 13C, to determine the connectivity and environment of atoms within the molecule.
- Mass spectrometry (MS): MS analyzes the mass-to-charge ratio of ions produced from the fragmentation of the molecule, providing information about its molecular weight and structural fragments.
Organic Compound Reactivity
Organic compounds exhibit a wide range of reactivities, which are influenced by their functional groups and molecular structures. Different classes of organic compounds undergo characteristic reactions due to the presence of specific functional groups.
Substitution Reactions
Substitution reactions involve the replacement of one atom or group of atoms in an organic compound with another atom or group. These reactions typically occur when an electrophile (electron-poor species) attacks a nucleophile (electron-rich species).
Common examples of substitution reactions include:
- Nucleophilic substitution: A nucleophile replaces a leaving group on an electrophile.
- Electrophilic substitution: An electrophile replaces a hydrogen atom on an aromatic ring.
Addition Reactions
Addition reactions involve the addition of one or more atoms or groups of atoms to a multiple bond in an organic compound. These reactions typically occur when an electrophile attacks a nucleophile with a double or triple bond.
Common examples of addition reactions include:
- Hydrogenation: Addition of hydrogen to a double or triple bond.
- Halogenation: Addition of a halogen to a double or triple bond.
Elimination Reactions
Elimination reactions involve the removal of two atoms or groups of atoms from an organic compound to form a multiple bond. These reactions typically occur when a base abstracts a proton from a carbon atom adjacent to a leaving group.
Common examples of elimination reactions include:
- Dehydration: Removal of water from an alcohol or an amine.
- Decarboxylation: Removal of carbon dioxide from a carboxylic acid.
Oxidation-Reduction Reactions
Oxidation-reduction reactions involve the transfer of electrons between two species. In organic chemistry, oxidation typically refers to the loss of electrons, while reduction refers to the gain of electrons.
Common examples of oxidation-reduction reactions include:
- Combustion: Oxidation of an organic compound with oxygen to produce carbon dioxide and water.
- Reduction of aldehydes and ketones: Reduction of a carbonyl group to an alcohol using a reducing agent.
Organic Compound Applications
Organic compounds find extensive applications in various industries, owing to their diverse properties and reactivity. Their versatility makes them essential components in numerous products and processes.
In the pharmaceutical industry, organic compounds serve as the foundation for a wide range of drugs. These compounds can be tailored to target specific biological processes, making them indispensable in treating various diseases. For instance, aspirin, an organic compound derived from salicylic acid, is a widely used pain reliever and anti-inflammatory medication.
Food Industry
Organic compounds play a crucial role in the food industry, contributing to taste, texture, and preservation. Sugars, such as glucose and fructose, provide sweetness, while fats and oils contribute to texture and flavor. Additionally, organic compounds like preservatives help extend the shelf life of food products by inhibiting microbial growth.
Materials Industry
Organic compounds are vital in the production of various materials. Plastics, made from polymers like polyethylene and polypropylene, are ubiquitous in packaging, construction, and automotive industries. Synthetic fibers, such as nylon and polyester, are used in clothing, carpets, and other textiles.
Energy Industry
Organic compounds serve as fuels and energy sources. Fossil fuels, including coal, oil, and natural gas, are primarily composed of organic compounds and provide a significant portion of the world’s energy needs. Biofuels, such as ethanol and biodiesel, derived from renewable organic sources, offer sustainable alternatives to fossil fuels.
FAQ Summary: Class Of Organic Compounds Crossword
What is the IUPAC nomenclature system?
The IUPAC nomenclature system is a standardized set of rules for naming organic compounds. It assigns unique, systematic names to compounds based on their structure and functional groups.
How are organic compounds classified?
Organic compounds are classified based on their functional groups, which are specific arrangements of atoms that determine their chemical properties. Common functional groups include alkanes, alkenes, alkynes, alcohols, aldehydes, ketones, carboxylic acids, and esters.
What are the different methods used to identify organic compounds?
Organic compounds can be identified using various techniques, including spectroscopy (IR, NMR, MS), chromatography (GC, HPLC), and chemical tests. Each technique relies on specific principles to determine the structure and identity of an organic compound.